Crystal of Jewel

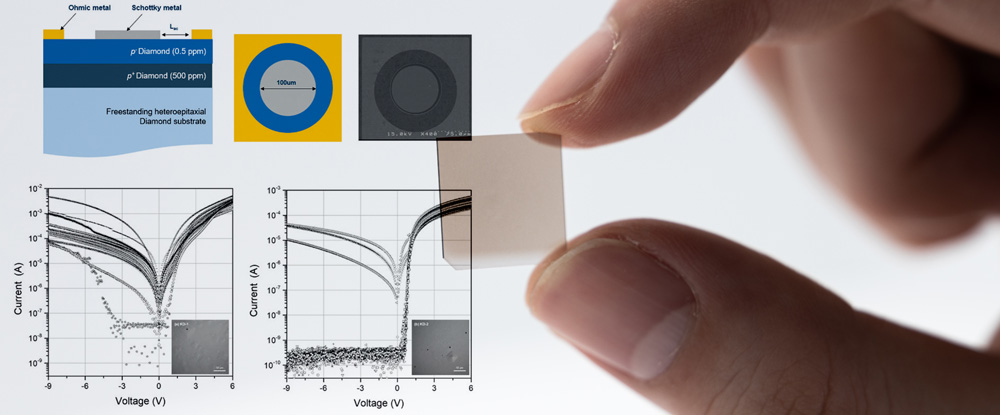
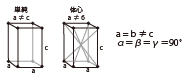
Every crystal is formed so that the atoms are orderly lined up in accordance with certain rules. The crystal of which atomic arrangement is located in a line with the fixed rule forms the three dimensional structure in order, which is surrounded by a plane. In some case, Opal is an amorphous material that the atoms are lined at random among of it. Why it becomes three-dimensional structure is that has something to do with the symmetrical property of the crystal that a plane surface, straight line and a point becomes symmetric. The crystal makes a form becoming symmetric and has some axes (crystalline axis) binding aspects to face each other in the internal center together. By the length of this crystalline axis and an angle to intersect, it can divide into seven kinds of crystals classification.
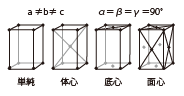
Adding four kinds way of thinking of lattices (Primitive, Base-centered, Face-centered and Body-centered) to this, and an identical points can be arranged spatially to produce 14 types of regular pattern (spatial sequence of the constitutive particle of the crystal). These 14 space lattices are known as 'Bravais lattices'.
Table of contents [close]
- 1. Crystals classification and Bravais lattices
- 2. Kinds of Crystal
- 2.1. (1) Covalent binding: a crystal in which all the atoms share the mutual electron and combine. Typically gives rise to a huge molecule.
- 2.2. (2) Ionic crystals: a crystal in which a positive ion and negative ion are combined.
- 2.3. (3) Metallic crystals: Metallic ion joined bond together was madein order
- 2.4. (4) Molecular crystals: the crystal which the molecule arranged regularly are lined up in order and what was made by the crystal which works between among molecules.
Crystals classification and Bravais lattices
| Crystal system | Bravais Lattices | Mineral | Color | Applications |
|---|---|---|---|---|
| Trigonal (Rhombohedral) |
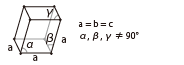
|
Sapphire Ruby |
Colorless (no impurity) Red |
Wafers, Watch parts, Scalpel |
| Cubic |
|
Rutile | Pale yellow | Isolator |
| Cubic |
|
Garnet LuAG(Lu3A15012) |
Black (Infrared transmission) Colorless to Pale yellow/Palegreen |
Isolator Scintillator |
| Spinel | Various | Watch parts | ||
| Diamond | Colorless | Scriber, Abrasive | ||
| Silicon | Blue-grey | Wafer | ||
| Boron nitride(cBN) | Pale yellow | Abrasive | ||
| Silicon carbide(SiC) | Pale yellow to Black green | Abrasive | ||
| Triclinic |
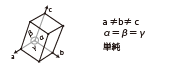
|
GPS(Gd2Si207) | Colorless | Scintillator |
| Monoclinic |
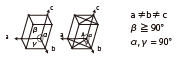
|
Gallium oxide | Colorless | Wafer |
| Hexagonal |
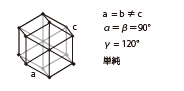
|
Gallium nitride | Cololess to Pale yellow | Wafer |
| Quartz | Colorless | Wafer | ||
| Orthorhombic |
|
YAP(YAI03) | Colorless | Scintillator |
Kinds of Crystal
There are four kinds of crystal depending on how an atom is tied to the crystals.
(1) Covalent binding: a crystal in which all the atoms share the mutual electron and combine. Typically gives rise to a huge molecule.
The melting and the boiling points are high. No electrical conductivity (however, except for black lead) Example diamond, black lead and Silicon dioxides.
(2) Ionic crystals: a crystal in which a positive ion and negative ion are combined.
The melting point and the boiling are high. No electrical conductivity (there being a liquid and solution).
Example sodium chloride (salt), a cesium chloride.
(3) Metallic crystals: Metallic ion joined bond together was madein order
There are the melting point and the boiling point high and low. High thermal conductivity and electrical conductivity.
Example, gold, iron and copper, almost all metal belongs to these v classification
(4) Molecular crystals: the crystal which the molecule arranged regularly are lined up in order and what was made by the crystal which works between among molecules.
Example iodine, dry ice and naphthalene.